Respiratory distress in dogs and cats can be caused by several conditions, and rapid identification of the cause is paramount to increasing the likelihood of successful treatment. A brief but focused physical examination and sound knowledge of the physiology of the respiratory system facilitates rapid localisation of signs. From here, a list of differential diagnoses can be created and treatments undertaken. Diagnostic testing often needs to be staged for the safety of the patient, and proficiency in performing and interpreting point of care tests (focused ultrasound, minimum database) can yield valuable information in a short amount of time to enable appropriate intervention.
Definitions
Tachypnoea (rapid breathing) can be the result of pain, stress, anxiety, excitement or respiratory pathology. Dyspnoea (discomfort or distress associated with breathing) is almost always associated with respiratory pathology, although apparent respiratory distress can be caused by non-respiratory conditions. Tachypnoea is a compensatory mechanism for metabolic acidosis. Kussmaul breathing is a specific deep, slow, laboured breathing pattern that has been described in patients with diabetic ketoacidosis. Electrolyte disturbances (hypokalaemia and hypocalcaemia) and hypoglycaemia can also have an impact on respiratory muscle function. Cats with hyperthyroidism can be tachypnoeic as a result of an increased basal metabolic rate. Metabolic conditions can be quickly ruled out with blood work, which should be obtained in a non-stressful manner.
Physiology
The respiratory system comprises of the upper (nasal cavity, nasal sinuses, nasopharynx, larynx and trachea) and lower (mainstem bronchi, bronchioles) airways, pulmonary parenchyma, pleural space and thoracic wall. The respiratory cycle involves active inspiration and passive exhalation. Various conditions impair ventilation (movement of air into and carbon dioxide out of the lungs) and oxygenation (gas exchange at the level of the alveoli). Hypoxaemia and severe hypoxaemia develop when the partial pressure of oxygen in arterial blood (PaO2) is less than 80 mmHg and less than 60 mmHg respectively. Causes of hypoxaemia include a low fraction of inspired oxygen (FiO2), global hypoventilation, right-to-left shunt, diffusion impairment and ventilation-perfusion mismatch. In clinical practice, ventilation-perfusion mismatch is most common (Tong and Gonzalez, 2020). Hypoventilation causes increased partial pressure of carbon dioxide (PaCO2) and can be the result of central nervous system disorders, neuromuscular disorders, chest wall abnormalities, obesity and obstructive airway processes.
Preparedness
The entire team should be trained to recommend an owner bring their pet in immediately if they are concerned about respiration. There are varying interpretations of laboured breathing in the home, and delays in treatment will negatively impact outcomes (American Animal Hospital Association, 2023). A ‘consent to treat’ or ‘STAT estimate’ can be helpful to allow teams to provide instant care. The term STAT, from the Latin word ‘statim’, is commonly used in healthcare and denotes the need for immediate attention. Communicating the urgency to an owner in an efficient way can help facilitate rapid and life-saving treatment. The ability to provide oxygen supplementation in a non-stressful way should be available. A clear triage area, fully stocked crash cart, intravenous catheter set up and emergency kits (thoracocentesis, temporary tracheostomy) should be easily accessible.
Initial assessment
The ABC of emergency triage always applies; is the airway patent, is the patient breathing and do they have circulation? If the answer is no, intubation and cardiopulmonary resuscitation in accordance with the RECOVER guidelines must be initiated (Burkitt-Creedon et al, 2024). If a patient has upper airway dyspnoea, a complete obstruction should quickly be ruled out. If an obstruction is present, a patent airway must be secured immediately. Clinicians should be familiar with techniques for a difficult intubation and have the necessary tools available.
The breathing pattern and body position should be quickly assessed, and any audible noise noted. Orthopnoea (altered body position with abducted elbows), neck stretching and a lateral body position can precede respiratory arrest. The use of extra thoracic muscles for respiration (flared nostrils) without expected thoracic wall movement can indicate hypoventilation, and a neurological examination should be performed.
Localisation
Respiratory disorders can be localised to the following categories: upper airway obstruction, lower airway obstruction, pulmonary parenchymal disease and pleural space disease. They can be differentiated from one another using a combination of observation and physical examination (Sigrist et al, 2011) (Table 1).
| Upper airway obstruction:
|
| Lower airway obstruction:
|
| Pulmonary parenchymal disease:
|
| Pleural space disease:
|
Physical examination
Physical examination of the respiratory system must include assessment of the mucus membranes and auscultation of the trachea, lungs and heart. Cyanosis is a late indicator of hypoxaemia, and assessment can be subjective and influenced by internal (anaemia) and external (lighting) factors. Auscultation can reveal increased lung sounds associated with lower airway and pulmonary parenchymal disease, or decreased lung sounds associated with pleural space disease. Severe consolidation can cause quieter lung sounds or a pleural ‘rub’ or ‘squeak’ at the end of inspiration. Upper airway obstructive diseases cause referred noise on auscultation and may mask lower airway sounds. Auscultation should be repeated once upper airway signs are controlled because of the high propensity for aspiration pneumonia or non-cardiogenic pulmonary oedema. A murmur, gallop or arrhythmia may indicate underlying cardiac disease. Signs of poor cardiac output, such as a reduced pulse quality, low body temperature, cool extremities, low blood pressure or tachycardia (or bradycardia in cats), can suggest congestive heart failure, or in some cases, shock associated with severe pneumonia. The use of additional physical examination techniques such as thoracic percussion, sternal compression (especially for cats) to assess thoracic wall compliance, and identifying the location of the apical beat can be helpful to narrow down differential diagnoses (Table 2).
| Localisation | Condition | Treatment considerations |
|---|---|---|
| Upper airway obstruction |
|
|
| Lower airway obstruction |
|
|
| Pulmonary parenchymal disease |
|
|
| Pleural space disease |
|
|
ARDS/ALI: acute respiratory distress syndrome/acute lung injury; FAAD: feline allergic airway disease; NCPO: non-cardiogenic pulmonary oedema; SP: sodium phosphate
Handling
Cats should be evaluated inside the carrier briefly to assess temperament and the degree of dyspnoea. If a cat is fractious, placing an Elizabethan collar over the head or placing a towel over the patient can be helpful before removing them from the carrier. A dyspnoeic cat should never be scruffed or restrained in lateral or dorsal recumbency. The environment should be quiet, free of barking dogs and other animals. If respiratory arrest is not imminent, it is recommended to administer butorphanol (0.2 mg/kg intramuscularly) and place in an oxygen cage at 40–60% FiO2 for 5–10 minutes. When the cat is calm and can be moved safely, staff should be ready to place an intravenous catheter, perform a physical examination and a focused point of care ultrasound in rapid succession if the cat is tolerant. Flow-by oxygen should be available, and handling aborted if the cat becomes stressed. A staged approach can include placing local anaesthetic cream on multiple intravenous catheter locations before removing the cat from an oxygen cage. Securing the cat close to the handler in a seated or sternal position, with a forelimb extended at the elbow for cephalic visualisation, or gently extending a hind limb for lateral or medial saphenous visualisation facilitates intravenous catheter placement. Avoid touching the feet, as this can increase stress. Flow-by oxygen should be held close by. Do not force an oxygen mask onto the face as this can worsen distress. Only personnel trained in animal handling and proficient in intravenous catheter placement should be handling dyspnoeic patients. Once intravenous access is obtained, other pharmacological therapies can be readily administered, and the cat can be placed back in oxygen. The same approach can be taken with small and medium dogs. In large dogs, nasal prongs, or a nasal cannula can facilitate immediate oxygen therapy while intravenous access is obtained.
History
A brief and clear history should be obtained at presentation, and a more thorough history obtained once the patient is stabilised. A history of trauma will increase the likelihood of a diaphragmatic rupture, traumatic pneumothorax or pulmonary contusions; a history of mitral valve disease and a cough will raise concern for congestive heart failure; a collapsing dog may have pulmonary hypertension; a febrile dog that has boarded recently may have infectious pneumonia; a dog that has been vomiting may have aspiration pneumonia; a cat with a history of coughing possibly has asthma and a cat that recently had fluid therapy may have heart failure.
If the patient is actively choking on a foreign object, intravenous propofol or alfaxalone can be administered to remove the object (with sponge forceps or large tissue forceps) and facilitate breathing. Older small breed dogs commonly have co-existing cardiac and respiratory disorders, and assessing stability compared with progression of heart disease can be helpful to determine which condition is causing the clinical signs that require treatment.
Signalment and pattern recognition
Brachycephalic breeds have anatomical abnormalities (stenotic nares, an elongated soft palate, everted laryngeal saccules, laryngeal oedema and/or collapse and, in some breeds, a hypoplastic trachea) that comprise brachycephalic obstructive airway syndrome. Laryngeal or nasopharyngeal masses cause upper airway obstructive dyspnoea that may be mistaken for lower airway obstructive disease. Cardiogenic pulmonary oedema is more likely in small breed dogs with degenerative valvular disease. Yorkshire terriers and Pomeranians are also predisposed to tracheal collapse, West Highland white terriers to pulmonary fibrosis, and Northern breeds (huskies/malamutes) are over-represented for spontaneous pneumothorax. Young cats are more susceptible to effusive feline infectious peritonitis. Familial hypertrophic cardiomyopathy has been recognised in Maine Coon and Ragdoll cats (Meurs et al, 2005; 2007).
Diagnostics
Pulse oximetry
Pulse oximetry measures oxygen saturation of haemoglobin in the blood (SpO2). An SpO2 of greater than 97% correlates with a partial pressure of oxygen in arterial blood (PaO2) of 80–100mmHg. An SpO2 of 95% and 90% are considered abnormal, as they correlate to PaO2 of 80mmHg and 60mmHg respectively (Haskins et al, 2015). Pulse oximetry has limitations, including the inability to differentiate oxyhaemoglobin from dyshaemoglobinemia, inaccuracies in the face of pigmentation, severe anaemia, hypoperfusion, excessive fluorescent light and motion artifacts (Jubran, 2015). Moreover, placement of the probe can exacerbate patient distress, rendering it less useful in acutely dyspnoeic patients. In the case of respiratory distress, patient assessment and physical examination should determine whether the patient requires oxygen therapy in place of a pulse oximeter.
Ultrasound
A point-of-care ultrasound is a non-invasive and essential technique that can be mastered in as few as 20 scans (McMurray et al, 2016). The thoracic-focused assessment for sonography of trauma or triage currently describes a 5-point protocol, performed in sternal recumbency. This includes bilateral chest-tube sites (between the 7th and 9th intercostal spaces on the caudo-dorsal aspect of the chest), pericardial sites (between the 5th and 6thintercostal spaces with the strongest heartbeat), and the subxiphoid view. Useful information includes identification of pleural effusion, pericardial effusion, the presence of a glide sign, alveolar infiltrates, subjective cardiac contractility, left atrium-to-aorta ratio, subjective vascular volume estimation (vena-cava diameter) and sub-pleural consolidations (Pelchat et al, 2020). The absence of a glide sign aids diagnosis of a pneumothorax in a patient with a high index of suspicion (Boysen and Lisciandro, 2013). It is helpful to differentiate ‘wet’ lungs (B-lines or lung rockets) from ‘dry’ lungs (glide sign and A-lines present) to know whether to treat with diuretics or antibiotics (Lisciandro and Lisciandro, 2021). In cats presenting with respiratory distress, a left atrium-to-aorta ratio≥1.5 is a good indicator for diagnosing congestive heart failure (Janson et al, 2020). The left atrium-to-aorta ratio can be measured in sternal recumbency in dyspnoeic cats (Ward et al, 2018; Burnotte et al, 2023), which allows a large amount of information to be obtained with minimal to no restraint.
Radiographs
Three-view thoracic radiographs provide important information. However, positioning a patient in respiratory distress for radiographs is life-threatening and should be avoided until the patient has been stabilised and a focused ultrasound has been performed. Pleural effusion will impair radiographic interpretation of the pulmonary parenchyma. A ventrodorsal view should never be obtained because turning a dyspnoeic patient on its back will cause further distress and panic. In most instances, a staged approach is safest, with a dorsoventral view always performed first for patient comfort and to reduce potential artefact from atelactasis, followed by the remaining orthogonal views up to 12 hours later once the patient is more stable.
Cervical radiographs may detect intraluminal masses or obstructions, but dynamic diseases such as tracheal collapse and mainstem bronchial collapse may not be seen or may be underestimated on radiography (Macready et al, 2007). Lower airway disease in dogs and cats is classically associated with a bronchial or bronchointerstitial pattern (Corcoran et al, 1995; Mantis et al, 1998). Non-cardiogenic pulmonary oedema is most likely to have a bilaterally symmetric distribution in the caudodorsal lung field (Bouyssou et al, 2017); however, more severe non-cardiogenic pulmonary oedema can manifest as an alveolar pattern. A perihilar to caudodorsal interstitial pattern is typical for cardiogenic pulmonary oedema secondary to left‐sided congestive heart failure in dogs. In most cases, this pattern is symmetrical, but it can be asymmetrical (Koster et al, 2023) or diffuse. Cats can have a variable distribution of cardiogenic oedema.
Pulmonary vein dilation reflecting venous congestion is another common radiographic finding in cardiogenic pulmonary oedema (Keene et al, 2019). A vertebral heart scale/score can be calculated from a right lateral thoracic radiograph to assess cardiac size. A vertebral heart scale of greater than 9.3 is highly specific for the presence of heart disease in cats presenting with acute respiratory distress (Sleeper et al, 2013). In dogs presenting with respiratory signs with increased vertebral heart scale, vertebral left atrial size is especially useful to help rule out congestive heart failure (Ross et al, 2023). In aspiration pneumonia, a predominantly alveolar (or interstitial) infiltrate is seen, with the right middle lung lobe usually most affected (Kogan et al, 2008). Fungal pneumonia typically has a diffuse or nodular interstitial pattern and hilar lymphadenopathy (Crews et al, 2008). Radiographic changes can lag up to 24 hours behind clinical signs (Gonzalez and King, 2019).
Blood work
A minimum database requires a very small amount of blood and can be performed at the time of intravenous catheter placement. This includes a packed cell volume, total solids, blood glucose and, if available, electrolytes and blood gas analysis. A complete blood count and chemistry panel (plus urinalysis and thyroid profile in older pets) will help to rule out metabolic disease and evaluate for systemic illness. This more comprehensive panel should be obtained once the patient is stabilised so that handling or restraint does not cause patient deterioration. An arterial blood gas will facilitate direct measurement of PaO2 and PaCO2 but specialised skills and materials are needed, and the patient may not tolerate the procedure. Caution must be exercised when performing venipuncture on dyspnoeic animals, as patient handling must be minimised.
Serum aminoterminal pro B-type natriuretic peptide
Serum aminoterminal pro B-type natriuretic peptide (serum pro-BNP) is a biomarker associated with atrial stretch, which is increased in cats with clinically significant heart disease. A quantitative serum pro-BNP enzyme-linked immunosorbent assay for plasma and pleural fluid has shown good accuracy in differentiating cardiac from non-cardiac causes of respiratory distress in cats, and a point of care serum pro-BNP performed on plasma, but not pleural fluid, distinguishes cardiac from non-cardiac causes of pleural effusion in cats (Hezzell et al, 2016). The accuracy on serum has been corroborated with further studies (Ward et al, 2018; Janson et al, 2020).
Treatment
Oxygen
Initial stabilisation principles are the same for all causes of dyspnoea; first and foremost, provide oxygen and sedation or anxiolysis. Oxygen can be provided in several ways (Tong and Gonzalez, 2020) (Table 3). It is imperative not to increase patient stress. Remove the rubber attachment from masks – a complete seal will worsen hyperthermia and hypercapnia. The degree of response to oxygen therapy will vary according to the cause of respiratory distress. High-flow oxygen therapy is a newer modality that provides oxygen support via heated humidified air delivered at flow rates up to 60 L/min with a FiO2 ranging from 21–100%, and is indicated in patients with hypoxaemia that have failed traditional oxygen therapy. It can be a viable alternative to mechanical ventilation in certain patients, or when mechanical ventilation is not an option. Several veterinary studies have compared high-flow oxygen therapy to traditional oxygen therapy and have shown significant improvement in PaO2 with minimal to no complications (Keir et al, 2016; Pouzot-Nevoret et al, 2019; Ramesh et al, 2021).
| Methods of oxygen supplementation | Mean fraction of inspired oxygen (%) |
|---|---|
| Flow-by oxygen | 24–45 |
| Face mask | 35–55 |
| Unilateral nasal catheter | 30–50 |
| Bilateral nasal catheter | 30–70 |
| Oxygen hood | 21–60 |
| Oxygen cage | 21–60 |
| High-flow oxygen therapy | 21–100 |
| Positive-pressure ventilation | 21–100 |
| Tong and Gonzalez, 2020 |
Sedation
Butorphanol (0.1–0.3 mg/kg intravenously or intramuscularly) is an effective sedative (Oyama, 2019). It is cardiovascularly safe and is short acting (1–2 hours) so can be re-dosed. It is also reversible with naloxone 0.01–0.02 mg/kg intravenously or intramuscularly (Plumb, 2015). Sedation can reduce respiratory drive, so be prepared to intubate patients that are on the verge of respiratory failure, but do not be afraid to provide sedation for its positive anxiolytic effects. Benzodiazepines are cardiovascularly sparing and completely reversible with flumazenil, but can cause paradoxical excitement and worsen respiratory distress if given alone. Acepromazine and dexmedetomidine are reserved for upper airway obstructive processes and should be avoided in pulmonary parenchymal disease because of the negative haemodynamic effects, including hypotension and bradycardia.
Specific treatments
Upper airway dyspnoea
Sedation
Control of anxiety in these patients is essential to break the cycle of inflammation and respiratory distress. In addition to butorphanol, acepromazine (0.005–0.02 mg/kg intravenously or 0.01–0.02 mg/kg intramuscularly) is an effective sedative; however, it can cause hypotension, has a long duration of action (4–6 hours) and cannot be reversed. Low doses should be used to begin with, and maximal intravenous effect is achieved in 15 minutes (Plumb, 2015). Dexmedetomidine is reversible (with atipamezole) and titratable with a short duration of action; however, it can produce bradycardia and hypotension and should be given at low doses (0.5–2 mcg/kg intravenously or intramuscularly in dogs) with careful monitoring (Plumb, 2015). In dogs, trazadone and/or gabapentin crushed and given via oral syringe if the patient can swallow and does not become more distressed, can be helpful for maintenance anxiolysis, but are not reversible. In cats, administering oral gabapentin once stable, to help with handling can be considered. Dyspnoeic patients should never be pilled, and rapid resolution of respiratory distress is essential. A summary of how to approach the dyspnoeic patient is provided in Box 1.
Summary of approach to the dyspnoeic patient
Cooling
In upper airway obstruction, hyperthermia develops very quickly. Hyperthermia worsens panting which, in turn, worsens upper airway swelling. It is imperative the patient is cooled down to break this cycle. The value of a tabletop or desk fan cannot be over-emphasised. Placing a fan in front of a panting brachycephalic or laryngeal paralysis patient can make the difference between intubating and not intubating. The fan must be in front of the face to allow for heat loss via evaporation over moist mucus membranes. Always lubricate the eyes to prevent irritation from airflow.
Corticosteroids
Injectable glucocorticoids are useful to reduce inflammation and oedema in patients with an upper airway obstruction, such as in laryngeal paralysis or brachycephalic airway syndrome. Dexamethasone sodium phosphate (0.1–0.15 mg/kg intravenously, subcutaneously or intramuscularly) has a quick onset (1–2 hours) and an intermediate duration of action (24–36 hours) (Plumb, 2015). A longer acting steroid is not recommended because the anti-inflammatory action needed is short term, and negative side effects could be prolonged. Gastroprotectants along with misoprostal should be administered if there has been recent use of non-steroidal anti-inflammatory drugs. Cats with occult heart disease could develop heart failure secondary to steroid use, and risks must be discussed with the owners (Smith et al, 2004).
Anti-nausea/prokinetics
It is prudent to administer maropitant as soon as possible to all patients with respiratory distress to minimise the likelihood of nausea and vomiting, which could worsen respiratory distress as a result of closure of the epiglottis during vomiting. This reduces oxygen delivery, worsens panic and increases the possibility of aspiration pneumonitis/pneumonia, which will further impair gas exchange. This is especially important in brachycephalic obstructive airway syndrome, as significant pleural pressure changes generated on inspiration against upper airway obstruction in these dogs can cause upper gastrointestinal tract disease such as oesophagitis, gastritis and duodenitis with resultant regurgitation or vomiting (Hoareau, 2019). Metoclopromide will assist gastric emptying from aerophagia and, in some cases, a nasogastric tube may be necessary to decrease gastric distension and minimise pressure on the diaphragm that can worsen respiratory distress. This should only be attempted if the patient is already intubated.
Intubation
Always be prepared for a ‘difficult intubation’, with small endotracheal tubes, a long laryngoscope blade, tongue depressor or malleable retractor to lift an elongated soft palate, suction to aspirate secretions and mucus, and stylets to help with intubation. It is crucial that these components remain in the crash cart, and a daily inventory is helpful.
If a patient's signs are consistent with complete upper airway obstruction (brachycephalic obstructive airway syndrome, laryngeal paralysis, tracheal collapse) prompt intubation is often necessary. Having a conversation with the owner early during communication is essential to ensure they are prepared for the possibility. Propofol or alfaxalone will facilitate intubation but should only be given to effect, and cardiovascular parameters monitored. The myocardial depressant effects of propofol are volume and speed dependent – give slowly even in an emergency to reduce deleterious effects. Always use a capnograph to assess ventilation of an intubated patient. If the patient is breathing room air, the endotracheal tube cuff can be deflated to allow for inspiration of mixed air. If attached to an anesthaesia circuit or receiving positive pressure ventilation, the endotracheal tube cuff should be inflated. Never be afraid to intubate a patient with upper airway distress, because once the airway is secured there is more time for assessment. Thoracic radiographs should always be performed to rule out concurrent lower airway disease. When ready to extubate, the upper airway patient should be normothermic, normoxaemic and normocapnoeic; and should have received the following medications: butorphanol (+/- gabapentin or trazadone administered via naso- or orogastric tube if intubated), maropitant +/- metoclopramide, gastroprotectants and antiinflammatory corticosteroids +/- bronchodilators. Always extubate in a quiet area with the tools needed for immediate reintubation (including induction agents) readily accessible. If intubation cannot be achieved, an emergency tracheostomy must be performed, and having the appropriate training and materials on hand is key.
If the patient requires intubation as a result of respiratory failure from pulmonary parenchymal disease, they will need to receive 100% FiO2 and positive pressure ventilation to maintain normoxaemia and normocapnoea. In severe acute cardiogenic pulmonary oedema, it is possible that a combination of diuretics, ‘dumping’ the patient and suctioning the endotracheal tube alongside a short period of positive pressure ventilation could allow for extubation and continued aggressive intensive care. However, a number of these patients will require mechanical ventilation if they were intubated for respiratory failure because of the ongoing need for positive end expiratory pressure and 100% FiO2 to maintain normoxaemia. To keep a patient intubated while assessments are carried out, benzodiazepines, butorphanol or dexmedetomidine as boluses or continuous rate infusions can be used. Avoid using gaseous anesthaesia, as isoflurane and sevoflurane have deleterious cardiovascular effects (Hettrick et al, 1996).
Lower airway obstructive disease
Brochodilators
In lower airway obstructive processes like bronchitis and asthma, beta-2 adrenergic receptor agonists such as terbutaline (0.01 mg/kg intramuscularly) or albuterol (1–2 puffs: 90 mcg metred dose) often result in rapid improvement of dyspnoea within 5–15 minutes (Sharp, 2014). Patients with tracheal collapse often have concurrent bronchitis or some degree of chronic bronchial disease, so bronchodilators are usually also warranted in those instances. Methylxanthines such as aminophylline (4–6 mg/kg slow intravenous infusion) or theophylline (5–10 mg/kg orally every 12 hours) may or may not be helpful.
Pulmonary parenchymal disease
Congestive heart failure
The goals of therapy include decreasing preload, decreasing afterload and enhancing contractility. Furosemide (2 mg/kg intravenously or intramuscularly) followed by 2 mg/kg intravenously or intramuscularly hourly until the patient's respiratory signs are substantially improved or a total dosage of 8 mg/kg has been reached over 4 hours is the mainstay of treatment. For severe pulmonary oedema, furosemide also may be administered as a continuous rate infusion (0.66–1 mg/kg/hour) after the initial bolus. Always provide the patient with access to water. Renal function and electrolytes should be monitored. Pimobendan (0.25–0.3 mg/kg every 12 hours) should be given as soon as possible to dogs. Dobutamine (2.5–10 μg/kg/minute as a continuous rate infusion in dogs; 2.5–5 μg/kg/minute in cats), starting low and increasing the dosage incrementally may be used in addition to the above treatments. Continuous electrocardiographic monitoring is recommended because of the possibility of arrythmias, especially in cats. A continuous rate infusion of sodium nitroprusside (1–15 μg/kg/minute for dogs and 0.5–2 μg/kg/minute for cats) for up to 48 hours can be useful for life‐threatening pulmonary oedema. Blood pressure should ideally be monitored, but the benefit must be weighed against handling the patient and causing an unhaired or shaved area of skin, can be used for the first 24–36 hours of hospitalisation. Angiotensin-converting enzyme inhibitors should not be given in the acute setting because of the rist of hypotension (Keene et al, 2019).
Pneumonia
If there is a high index of suspicion for, or confirmation of, bacterial pneumonia, broad spectrum empiric antibiotics should be started as soon as possible. A delay in antibiotic therapy can lead to sepsis and the need for mechanical ventilation (Schulze and Rahilly, 2012). Doxycycline is a reasonable empiric choice for dogs or cats with mild pneumonia suspected to be related to Bordatella bronchiseptica or Mycoplasma spp. with no systemic signs or respiratory distress. If the patient requires hospitalisation, a beta-lactam antimicrobial like ampicillin, ampicillin-sulbactam or the first-generation cephalosporin cefazolin might be sufficient. If clinical findings suggest systemic illness or sepsis, concurrent administration of enrofloxacin or marbofloxacin is recommended (Lappin et al, 2017).
Sterile chemical aspiration pneumonitis predisposes to secondary bacterial infection with opportunistic pathogens from the normal pharyngeal flora (Tart et al, 2010). The most common isolates are Klebsiella spp. and Escherichia coli, with Staphylococcus spp. and Streptococcus spp. occurring less frequently (Proulx et al, 2014). If an endotracheal or trans-tracheal wash can be safely performed, an in-house cytology can confirm the presence of and the morphology of bacteria and de-escalation can be based on culture. Geographic, community and patient-specific factors impacting antimicrobial resistance patterns should be considered. Viral, fungal, parasitic and protozoal pneumonia can also occur.
Pulmonary contusions
Treatments for pulmonary contusions include oxygen therapy, butorphanol, anxiolysis and time. Pulmonary contusions can worsen over the first 24–48 hours before improvement is noted (Ganie et al, 2013). Treatment for concomitant shock and other injuries is needed. Fluid therapy poses a unique challenge because of the increased vascular permeability in the lungs and concern for fluid overload. Fluid resuscitation should always aim to restore and normalise systemic blood pressure, while minimising risk of fluid overload. These patients should be monitored extremely carefully.
Non-cardiogenic pulmonary oedema
Depending on the cause (electrocution, drowning, strangulation, upper airway obstruction, seizures) non-cardiogenic pulmonary oedema can also respond to oxygen and anxiolysis over a period of 24–72 hours. Furosemide +/- bronchodilators may or may not be helpful. Some of these patients may require intubation and mechanical ventilation.
Pleural space disease
If pleural space disease is compromising respiratory function, thoracocentesis is essential (Box 2). A pre-made kit is useful to ensure preparedness (Figure 1). Butorphanol alone can be sufficient +/- local anesthaesia. In cats, the addition of midazolam (0.1–0.3 mg/kg intramuscularly or intravenously) +/- ketamine (0.5–2 mg/kg intravenously or intramuscularly) to butorphanol can be helpful to facilitate thoracocentesis. Midazolam can cause transient exciteability and should not be given as a sole agent. Always have reversal agents pre-drawn. Propofol and alfaxalone are induction agents and should be avoided in respiratory distress unless the patient is intubated. Pleural fluid can be evaluated in-house to differentiate between a transudate, modified transudate or exudate, and a cytology should be performed to look for the presence of bacteria or neoplasia. Fluid can be submitted externally for cytology and fluid analysis, as well as bacterial culture (aerobic and anaerobic) if indicated. Once therapeutic thoracocentesis has been performed, radiographs to evaluate the pulmonary parenchyma can be performed. Pleural effusion secondary to heart failure will not respond to diuretics alone. If pleural effusion is purulent (pyothorax) needle thoracocentesis may be unsuccessful as a result of fluid viscosity, necessitating the placement of thoracostomy tubes. Low profile over-the-wire thoracic drainage tubes can be placed under sedation in these cases.
Process of performing thoracocentesis
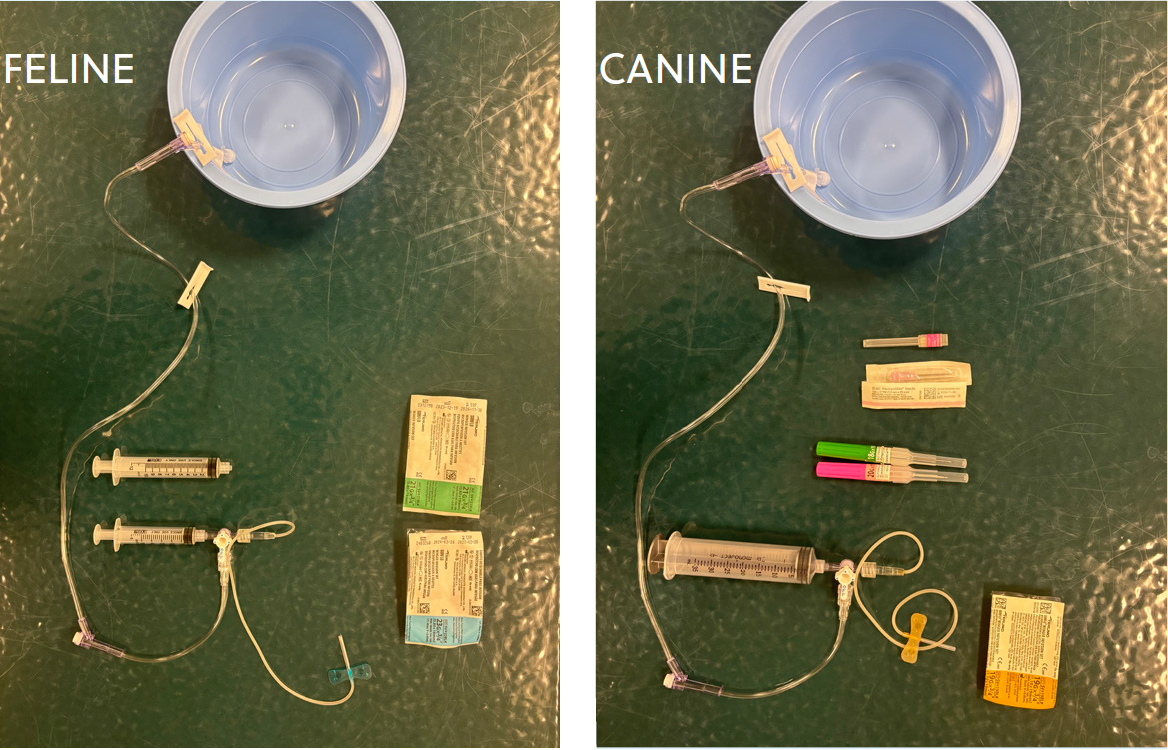
Conclusions
Team communication and education around how to safely manage the dyspnoeic patient are crucial to be prepared for this emergency. The over-riding goal is to minimise further stress to the patient by rapidly identifying the source of the problem with as little handling as possible. Close monitoring and reassessment is important to evaluate response to treatment and make additional treatment choices accordingly. If the patient is not responding to initial treatments as expected, reassessment or consideration of concurrent conditions is indicated, and further investigation may be needed. Alongside an accurate physical examination, bedside diagnostic tests (focused ultrasound, minimum database) help to rule in or out several conditions. Once the source is localised, treatments should be performed to facilitate rapid stabilisation. Prognosis depends on the underlying disease process, but being able to confidently manage dyspnoea in the immediate setting allows more time to perform further tests to gain information. Respiratory disease is often dynamic and explaining that to the owner helps set expectations appropriately.


