Peripheral nerve tumours can develop from Schwann cells, perineural cells or intraneural fibroblasts (Vail et al, 2020). The terminology that reflects cellular origin is not often used in veterinary medicine because it is difficult to determine the tissue of origin. Instead, these tumours are classified as malignant or benign based on microscopic evidence of malignancy (Vail et al, 2020).
Peripheral nerve sheath tumours occur most frequently in medium- to large-breed, middle-aged to older animals. The trigeminal nerve is the most commonly affected cranial nerve by these tumours. Reported clinical signs in dogs with trigeminal nerve sheath tumours include unilateral masticatory muscle atrophy and reduced or absent facial and corneal sensation. Neurological signs associated with brainstem compression may occur, but metastatic disease is rare. Magnetic resonance imaging scans of peripheral nerve sheath tumours will show trigeminal nerve thickening in up to 50% of patients (Vail et al, 2020). Differential diagnoses which may mimic peripheral nerve sheath tumours in advanced imaging include idiopathic, infectious and inflammatory neuropathies, lymphoma affecting the trigeminal nerve (lymphoneuromatosis) and hypertrophic ganglioneuritis (Vail et al, 2020).
Definitive diagnosis of peripheral nerve sheath tumours is challenging, and diagnosis is often presumptive. Surgical biopsy of trigeminal peripheral nerve sheath tumours are difficult to obtain because of the anatomical difficulties in accessing the nerve. A surgical approach via craniotomy to obtain a biopsy and to remove the lesion has been described in the literature (Bagley et al, 1998). However, this technique is invasive and is associated with significant postoperative complications, including neurological signs such as nystagmus, seizures and hemiparesis (Bagley et al, 1998). One case report described a peripheral nerve sheath tumour diagnosed via a surgically-obtained wedge biopsy of the infraorbital branch of the trigeminal nerve in a dog (James et al, 2020).
In human medicine, the expression of the immunolabel S-100 is used to differentiate between spindle cell tumours of neural and non-neural origin, and is a sensitive but non-specific marker for peripheral nerve sheath tumours (Gaitero et al, 2008). In veterinary medicine, S-100 cannot distinguish conclusively between these two categories of sarcomas (Meuten, 2020). Also, variable to negative immunoreactivity with S-100 is observed in canine peripheral nerve sheath tumours, especially in undifferentiated neoplasias (Meuten, 2020).
To the author's knowledge, this is the first report of a canine trigeminal nerve sheath tumour diagnosed with confidence using a minimally invasive method – tru-cut biopsy of the infraorbital nerve.
Case description
A 7-year-old female neutered Bull Terrier was referred for a chronic left temporal muscle atrophy and an 8-week history of tendency to rub the left side of the face. The patient was on empirical treatment with amoxicillin-clavulanic acid (20 mg/kg orally twice a day for 14 days) before consultation.
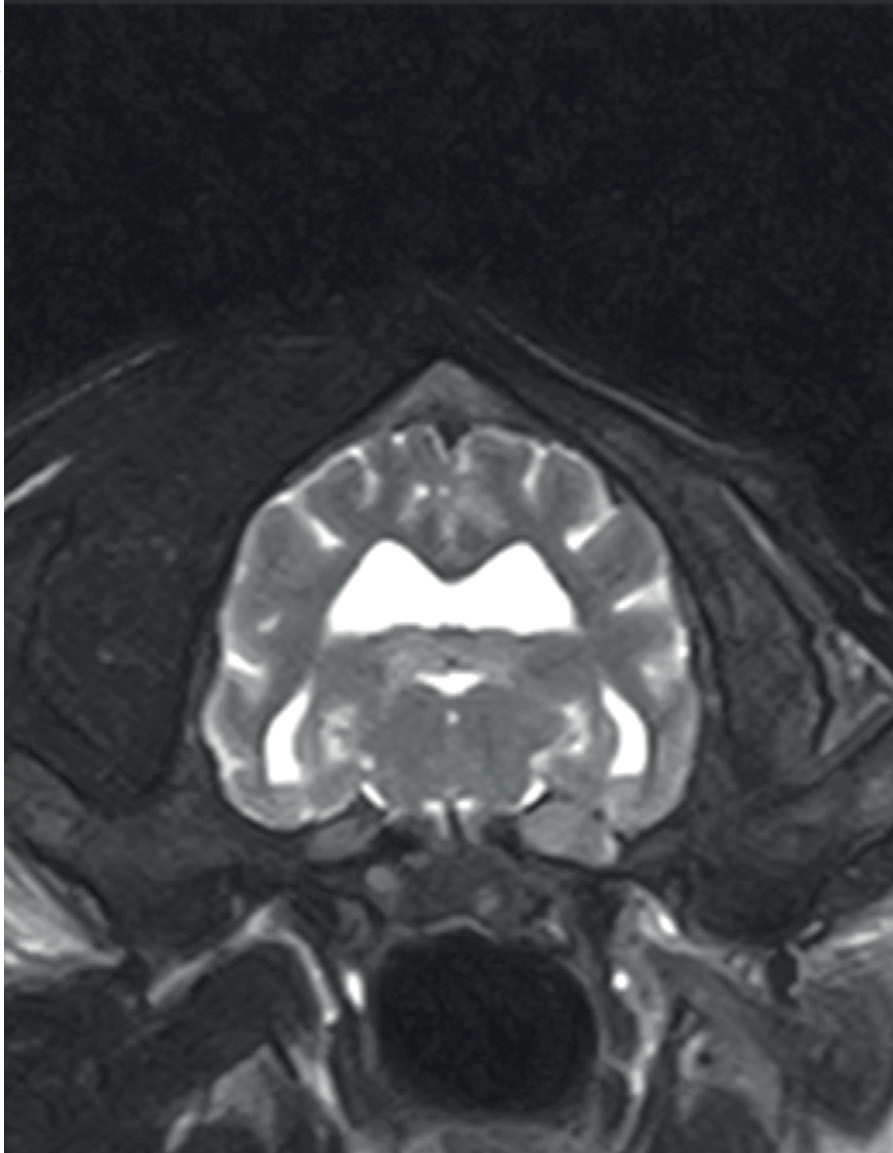
On physical examination, a small, firm, non-mobile and painless subcutaneous mass was detected on palpation of the left maxilla at the level of the infraorbital foramen, extending towards the fossa canina/ossis maxilla (Figure 1). Neurological examination revealed normal mentation, posture and gait. There was mild exophthalmos of the left eye, marked atrophy of the left temporalis and masseter muscles, and reduced sensation in the left nostril. The rest of the examination, including postural reactions and spinal segmental reflexes, was normal. There were no signs of pain and no restriction of movement on temporo-mandibular joint manipulation.

Based on the patient clinical signs, history and examination, a lesion on the left trigeminal nerve affecting the mandibular and maxillary branches was suspected. Neoplasia was the main differential diagnosis; an inflammatory/idiopathic process (neuritis) or infectious process (toxoplasmosis, neosporosis) were not excluded, but were considered less likely.
Diagnostic investigations and outcome
The patient was admitted and complete blood count, serum biochemistry, electrolytes and thyroid function (including total T4 and thyroid-stimulating hormone) were assessed.
These showed mild neutrophilia (15.17x109/L; reference range 2.94–12.67), mild uraemia (10.6 mmol/L; reference range 3.1–10.1, mild hyperbilirubinaemia (8.9 umol/L; reference range <5.1) and mild elevation of the creatinine kinase (235 U/L; reference range 20–225). These results were considered non-significant. Full urinalysis including culture was within normal limits apart from a trace of proteinuria with a normal urine protein:creatinine ratio.
Magnetic resonance imaging of the head was performed under general anaesthesia in sternal recumbency using a 0.4 Tesla (Hitachi Aperto Lucent) scanner. The following multiplanar magnetic resonance sequences were performed:
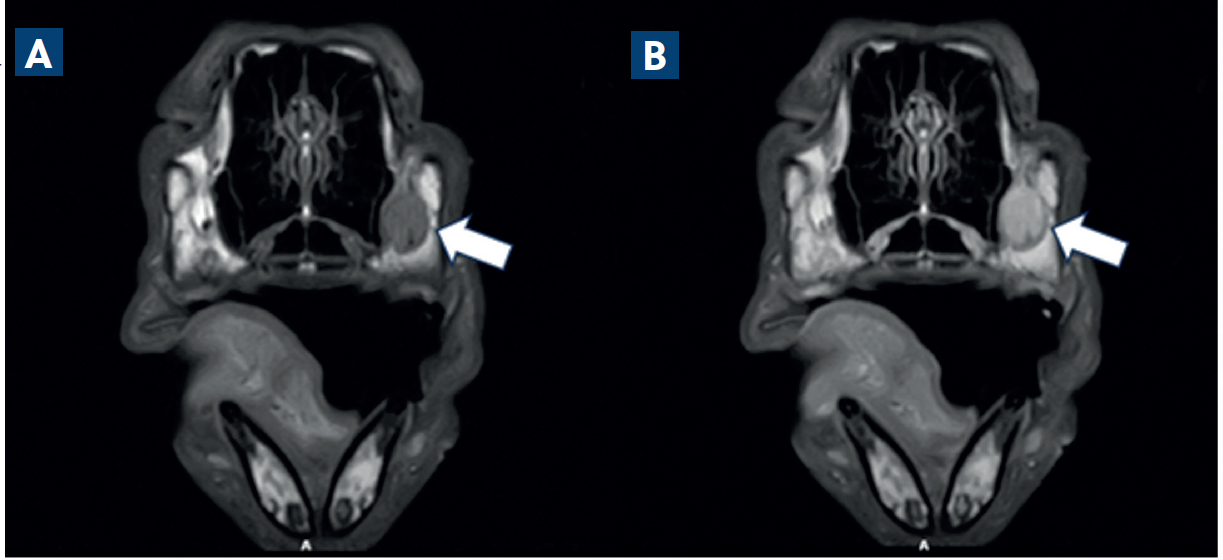
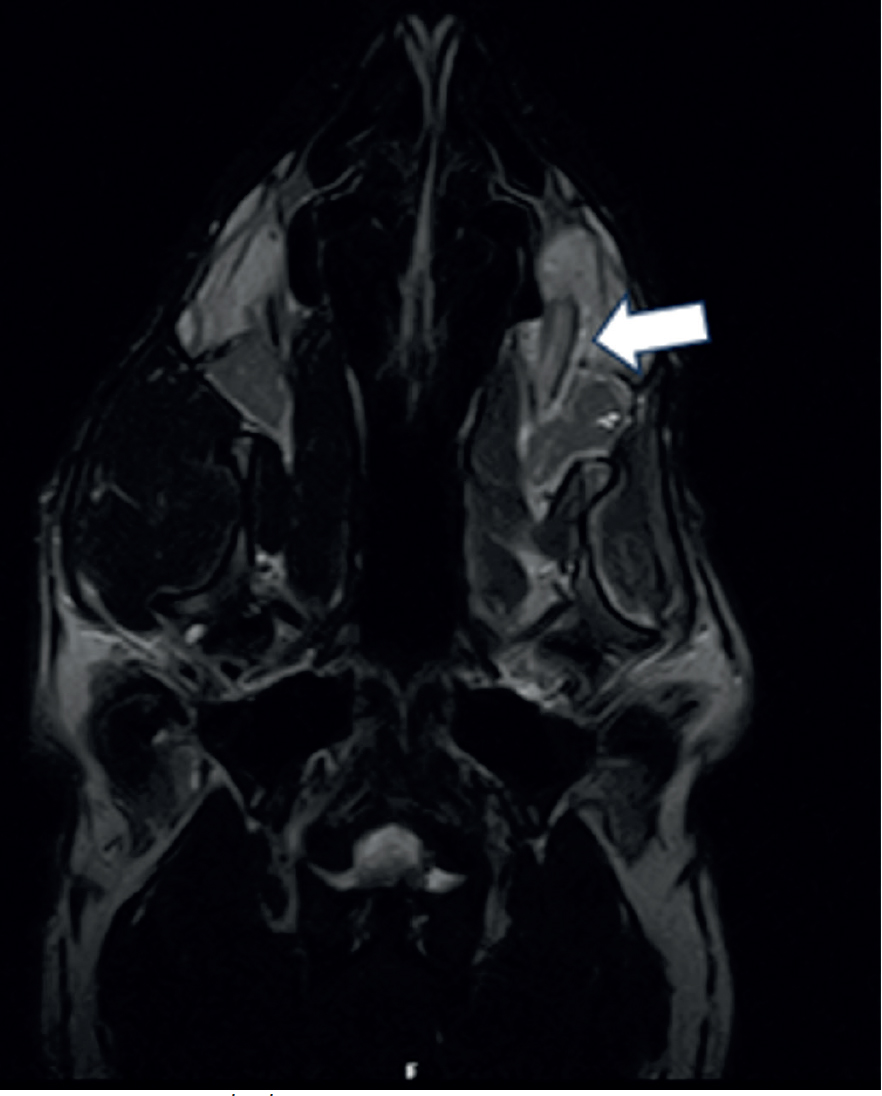
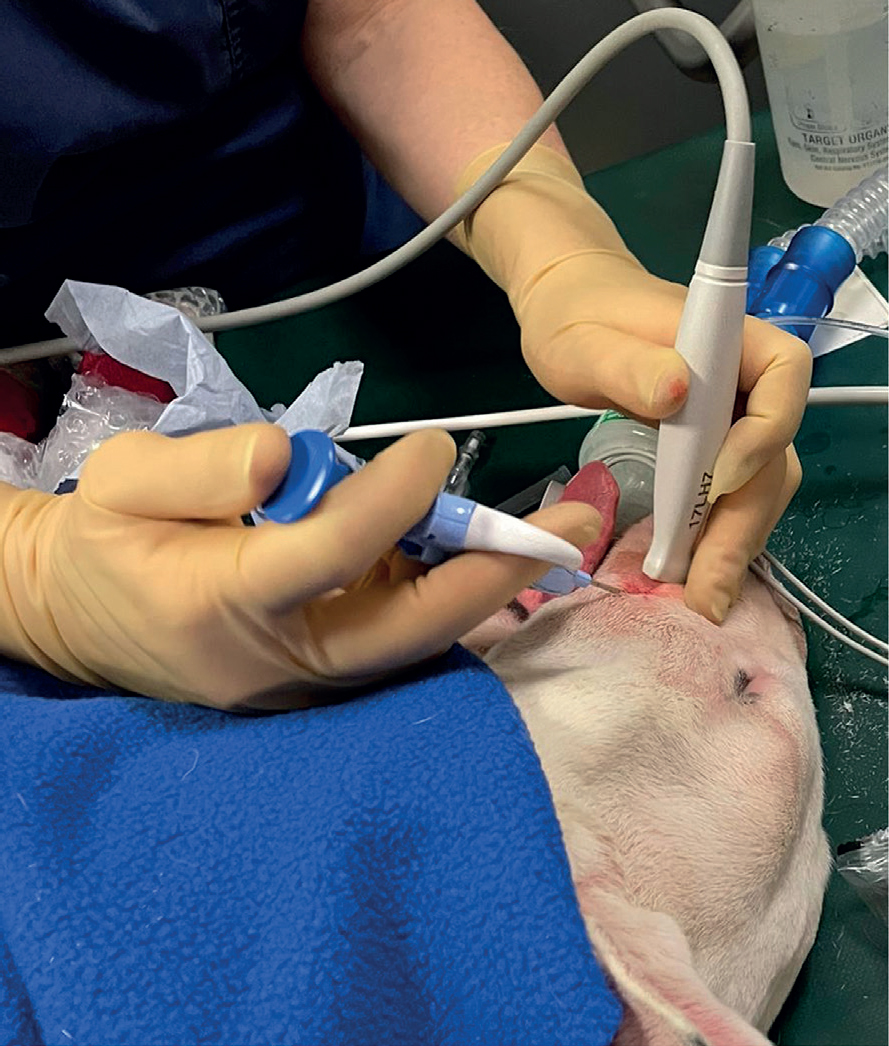
Magnetic resonance images confirmed the presence of a large trigeminal nerve mass on the left side (Figure 2). There was severe enlargement of the left trigeminal nerve, which was five times the diameter of the right trigeminal nerve (Figure 3). The abnormalities were extensive and involved the mandibular and maxillary branches. The abnormal changes extended rostrally to the left infraorbital nerve which resulted in enlargement of the infraorbital canal and remodelling of the bone (Figure 4). The lesion located on the left infraorbital nerve was consistent with the subcutaneous/soft tissue lesion located on the left maxillary bone. The enlarged trigeminal nerve's branches were slightly heterogeneous and increased in signal on the T2W images. The post-contrast images showed extensively marked, slightly heterogeneous contrast enhancement of the abnormal trigeminal nerve branches. Proximally, at the level of the left side of the brainstem, there was extension of the changes with focal, ill-defined T2W hyperintensity in the brainstem parenchyma at the origin of the trigeminal nerve. The rest of the brain and cranial nerves appeared normal. There was severe atrophy of the left masticatory muscles with diffusely increased signal on the T2W images and iso- to mildly increased signal intensity on the T1W images. The abnormal muscles showed mild homogeneous contrast enhancement. Mild atrophy of the caudal left part of the digastricus muscle was also reported. The cranial lymph nodes were normal on appearance and no other abnormalities were reported. Fine needle aspirates using 22G needles (Figure 5) and six biopsies (measuring 2x1x1 mm to 15x2x2 mm) using a 14G Tru-cut Soft Tissue biopsy needle were taken with ultrasound guidance from the enlarged left infraorbital nerve (Figure 6).
Cytology was supportive of a malignant neoplasia. A population of oval to spindloid cells exhibiting atypical features, such as moderate anisocytosis and anisokaryosis with occasional binucleated forms, was noted. A round cell morphology was also reported in some of the cells. The origin of these cells could not be confidently determined, and differential diagnoses included a poorly differentiated sarcoma or amelanotic melanoma.
Histopathology of the enlarged left infraorbital nerve revealed a non-encapsulated, moderately demarcated, locally infiltrative and poorly differentiated malignant neoplasm. The neoplastic cells were described as large, polygonal to spindle shape with moderately discrete cell borders, a small amount of eosinophilic cytoplasm and one nucleus. There were seven mitotic figures reported in 10 high power fields (per 2.37 mm2) and a small number of scattered apoptotic cells. The neoplastic cells were arranged in vague streams and sheets supported by small amounts of collagen which favoured a mesenchymal origin neoplasm rather than a round cell tumour. However, a melanocytic origin was not fully excluded. Therefore, further immunohistochemistry was requested to determine the origin of the described lesion.
Immunohistochemistry for vimentin, S-100, MelanA, PNL2, Iba1, CD3, CD20 and toluidine blue staining was requested to further investigate mesenchymal, peripheral nerve sheath, melanocytic and round cell origin (histiocytic, lymphoma and mast cell tumour) respectively. Most of the neoplastic cells (>90%) expressed strong cytoplasmatic staining for vimentin and no labelling for S-100, MelanA, PNL2, Iba1, CD3 or CD20. This supported a mesenchymal origin of a poorly differentiated neoplasm, consistent with a sarcoma. Negativity for PNL2, MelanA, Iba1, CD3 and CD20 did not support a melanoma, histiocytic origin tumour or lymphoma diagnoses. Negativity for S-100 did not exclude an undifferentiated peripheral nerve sheath tumour. Toluidine blue staining was negative, which excluded a mast cell tumour.
Infectious disease (such as neosporosis, toxoplasmosis or distemper virus) serology and polymerase chain reaction were not assessed as the cytology and histopathology samples were obtained during the work up, which did not raise any suspicion of an infectious disease.
Unfortunately, surgical excision was not a feasible treatment option for the described patient given the extent of the primary lesion. Radiation therapy was recommended in this case as it offers a safe treatment option with some evidence of benefit to a patient with a trigeminal peripheral nerve sheath tumour over palliative care (Hansen et al, 2016; Swift et al, 2017; Dolera et al, 2018). After consideration, the owners elected palliative treatment. Gabapentin (10mg/kg orally three times a day) and prednisolone (20mg/m2 daily for 7days, then reduced to 10mg/m2 daily ongoing) were prescribed. The tendency of rubbing the left side of the face improved but did not fully resolve after a few weeks of gabapentin therapy. The patient was lost to follow up shortly after.
Discussion
This is the first case report describing a canine peripheral nerve sheath tumour of the trigeminal nerve diagnosed using tru-cut biopsy and immunohistochemistry.
One study evaluated the use of tru-cut biopsy in maxillofacial pathology in humans, and no major complications were reported. The study showed that tru-cut biopsy minimises tissue trauma, reduces the risk of metastatic spread during the procedure and has a low complication rate (Kansagara et al, 2021).
Tru-cut biopsy offers other several advantages over open biopsy. It is a minimally invasive procedure that is quick and easy to perform, requiring no surgical preparation and carrying a lower risk of complications (Dupuy et al, 1998). While open biopsy can provide a larger tissue sample for histological evaluation, it is associated with a higher risk of complication, including bleeding, haematoma formation, infection and tumour dissemination (Dupuy et al, 1998).
Tru-cut biopsy is less painful, can obtain diagnostic samples from deeper, imaging-guided biopsies and results in fewer complications. Tru-cut biopsy is therefore considered a valuable diagnostic aid in the management of patients with head and neck lesions (Kansagara et al, 2021). A biopsy is only considered successful when diagnostic target tissue is obtained. Many pathologists consider core tissue obtained via tru-cut biopsy useful, as it preserves tissue architecture, allowing histopathological evaluation, including immunohistochemical analysis (Kansagara et al, 2021).
In human medicine, open biopsy is no longer justified in the diagnosis of maxillofacial pathology because of the high risk of tumour seeding, facial nerve injury, scarring and fistula formation (Kansagara et al, 2021). Open biopsy is also avoided as it may compromise subsequent surgery for definitive excision (Hems et al, 2024). While tru-cut biopsy is commonly used in human medicine for the diagnosis of peripheral nerve sheath tumours, as it offers higher sensitivity and specificity compared to cytology (with a retrospective analysis reporting a sensitivity of 95% and specificity of 97% in diagnosing musculoskeletal tumours, including Schwannomas; Seng et al, 2013), its application in veterinary medicine, particularly for trigeminal peripheral nerve sheath tumours in dogs, has not been extensively reported (Aref and Abizeid, 2018).
The dog described in this case report exhibited signs of left-sided facial hyperaesthesia. Facial hyperaesthesia has been described in dogs with peripheral nerve sheath tumours (Bagley et al, 1998; Milodowski et al, 2019; James et al, 2020); however, facial hypoalgesia associated with peripheral nerve sheath tumour is a more common clinical sign (Bagley et al, 1998; Swift et al, 2017). In human medicine, patients with trigeminal peripheral nerve sheath tumours often report facial numbness secondary to trigeminal nerve dysfunction, trigeminal neuralgia or a dysfunction of other cranial nerves because of tumour compression, and other symptoms as a result of increased intracranial pressure (Li et al, 2021). Medical therapy is the first line treatment option for neoplastic trigeminal neuralgia in humans. There are currently few approved medications for the treatment of this disease, including gabapentin. Pregabalin and levetiracetam are reported to be effective, but their use remains off-label (Allam et al, 2023). Surgical excision of encapsulated peripheral nerve sheath tumour through skin incision along the naso-labial fold has been described in human medicine (Kumar, 2015).
A combination of surgery with radiation therapy significantly improves local control compared to surgery alone in human patients with peripheral nerve sheath tumours. Patients treated with both modalities had a median local control time that was not reached, compared to 8.7 months for surgery alone (Roohani et al, 2023). With trigeminal tumours in particular, aggressive surgical resection followed by radiation therapy is associated with the best outcome because of the highly infiltrative nature of these tumours (Schmidt et al, 2013).
Research on the treatment of peripheral nerve sheath tumours in dogs indicates that radiation therapy, particularly stereotactic radiotherapy, provides significant survival benefits compared to palliative care and surgery alone. Stereotactic radiotherapy for trigeminal peripheral nerve sheath tumours resulted in improved survival and quality of life in treated dogs, with a reported median disease‐specific survival of 745 days (Hansen et al, 2016). Similarly, Swift et al (2017) reported that dogs with trigeminal peripheral nerve sheath tumours treated with a combination of therapies, including surgery and radiation, showed better survival rates, with stereotactic radiotherapy being particularly effective. The neurological intracranial signs of the irradiated dogs improved in 4 out of 5 dogs, and the overall median survival time reported was 441 days for the treated dogs, and 12 days for the dogs that received palliative treatment only.
A more recent study investigated the use of volumetric modulated arc radiotherapy for treating trigeminal peripheral nerve sheath tumours in dogs. Volumetric modulated arc radiotherapy allowed precise delivery of radiation, reporting a median overall survival of 950 days, with favourable outcomes in most cases (Dolera et al, 2018).
Advanced imaging is important in diagnosis and prognosis of canine trigeminal peripheral nerve sheath tumours. Dogs with trigeminal peripheral nerve sheath tumours had intracranial extension of the tumour and 81% of them had more than one branch affected (Swift et al, 2017). Furthermore, in a series of cases, dogs with trigeminal nerve tumours had mass lesions while dogs with neuritis had only diffuse enlargement of the nerve (Schultz et al, 2007).
As a result of the intracranial location, surgical biopsies and surgical excisions carry a high risk of complications and are not frequently performed in dogs. Therefore, most of the patients with trigeminal peripheral nerve sheath tumours would have only a presumptive ante-mortem diagnosis. In the case described here, it was feasible to sample the altered peripheral nerve because it was approachable at a location external to the skull (Figure 1). Infraorbital nerve surgical biopsy has previously been described in another case (James et al, 2020).
In dogs, benign peripheral nerve sheath tumours have been described as Schwannoma, neurofibroma, perineuroma and hybrid nerve sheath tumours (consisting of perineuroma in combination of Schwannoma and neurofibroma areas), although most canine nerve sheath tumours are malignant (Vandevelde et al, 2012). In one study, the malignant tumours were divided into conventional, divergent, perineural and epithelioid, with the most common variant being conventional (ie spindle cell) (Tekavec et al, 2022).
Histopathology is necessary to confirm a diagnosis of malignant peripheral nerve sheath tumour, as there is no single immunocytochemical marker that can distinguish malignant vs benign tumours (Meuten, 2020). On histopathology, most malignant peripheral nerve sheath tumours have cytological features including dense hypercellularity of pleomorphic spindle cells, with hyperchromatic, large, tapered nuclei with marked nuclear atypia. The most reliable histological criteria for the diagnosis of a peripheral nerve sheath tumour are cell crowding with high cell density, nuclear atypia and a high mitotic rate, but it may be difficult to differentiate between peripheral nerve sheath tumours and other undifferentiated or spindle cell neoplasias (Klopfleisch et al, 2013).
S-100 is a marker of Schwann cells, and in humans it is uniform and strong in all Schwannomas, while only 50–70% of human peripheral nerve sheath tumours express this marker (Gaitero et al, 2008). Canine peripheral nerve sheath tumours can lack expression of this marker, so the negativity for S-100 in the described case does not exclude a diagnosis of a peripheral nerve sheath tumour (van Stee et al, 2017). There is a consensus in the literature that any sarcoma with intrinsic involvement of a major nerve, without a specific line of differentiation (haemangiosarcoma, synovial sarcoma etc) should qualify as a malignant peripheral nerve sheath tumour (Rodriguez et al, 2012).
Variable to negative immunoreactivity can also be seen with CD57, GFAP, laminin and collagen IV (Meuten, 2020) markers, which were not used in this case. Vimentin positivity confirms the diagnosis of sarcoma. Claudin-1 can be expressed in human and canine peripheral nerve sheath tumours but not in Schwannomas, and can be used to diagnose benign canine peripheral nerve sheath tumours (Jakab et al, 2012). Sox-10 is also a marker which can be useful to confirm the neural crest origin of the tumour (Meuten, 2020).
In human medicine, the World Health Organization lists the grading and the histopathological subtypes of peripheral nerve sheath tumours (Louis et al, 2007). Higher grades are associated with rapid disease progression, infiltrative nature and decreased survival (Ziadi and Saliba, 2010). A similar histopathological prognostic grading system for peripheral nerve sheath tumours in veterinary medicine has been proposed by Tekavec et al (2022). In the present case, the reported peripheral nerve sheath tumour would be classified as intermediate grade. However, only biopsies were obtained for histopathology, and the concordance in grade between excisional and pre-treatment biopsies is reported to be only 59% in canine soft tissue sarcomas (Perry et al, 2014).
Conclusions
This is the first case report describing a malignant peripheral nerve sheath tumour diagnosed in a dog via tru-cut biopsy sampling, which is a less invasive alternative to surgical biopsy. Tru-cut biopsy can reduce morbidity, shorten procedural time and is generally more cost-effective in most veterinary facilities. This case also emphasises the importance of performing advanced imaging of the trigeminal nerve and its branches, including the infraorbital nerve, to identify mass-like lesions suitable for biopsy.


